Subprogramme 3: Integrated Biocatalysis & Crystallization
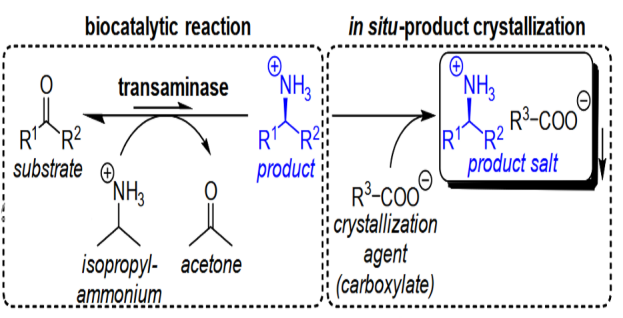
Subproject SP 3 aims to enzymatically synthesize and obtain enantiopure α-amino acids and β-amino alcohols at preparative scale, two substance classes that are frequently applied as intermediates during the synthesis of valuable agrochemicals and pharmaceuticals. This includes the selection and use of suitable biocatalysts and their corresponding preparations within the integrated synthetic route. Since these conditions influence directly the solubilities of all reactants, the necessary investigations involve the collection of fundamental physicochemical properties of the target compounds, as they are isolated directly from aqueous reaction solutions.
For amino acids, direct crystallization during the selected crystallization conditions is preferred, whereas suitable crystallization agents are required for amino alcohols to crystallize these typically hydrophilic products in the form of a salt. The aim of reactive crystallization in the context of the biocatalysis is to overcome some of the processes’ limitation such as low yield or reaction rate by removing the product(s) from the reaction mixture via crystallization and hereby shifting the equilibrium to the product side. Another aspect is to integrate the synthesis and crystallization of that compound to improve the general performance of such a process by e.g. reduced number of apparatuses and downstream processing steps. Necessary investigations will first be conducted in idealized aqueous systems to establish necessary methods which will be adapted later to real synthesis systems. The investigations will start with L-homophenylalanine, an α-amino acid with multiple applications in pharmaceutical industries. Later, different derivates and selected examples of β-amino alcohols will follow.
